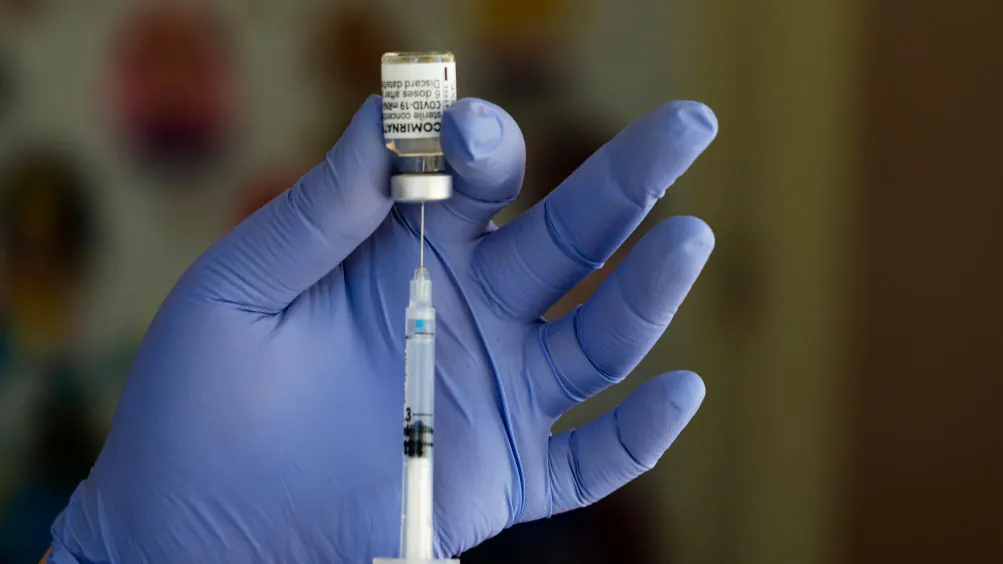
The Food and Drug Administration issued full approval to the Pfizer-BioNTech COVID-19 vaccine on Monday morning, marking the first time a vaccine against the disease caused by the novel coronavirus pandemic has been given the stamp of approval from regulators.
The vaccine, which was granted FDA emergency use authorization in December, will now be marketed under the name Comirnaty for Americans 16-years-old or older.
“The FDA’s approval of this vaccine is a milestone as we continue to battle the COVID-19 pandemic. While this and other vaccines have met the FDA’s rigorous, scientific standards for emergency use authorization, as the first FDA-approved COVID-19 vaccine, the public can be very confident that this vaccine meets the high standards for safety, effectiveness, and manufacturing quality the FDA requires of an approved product,” said Acting FDA Commissioner Janet Woodcock in a statement.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” she said.
Data from the Centers for Disease Control and Prevention shows that 170 million people in the United States have already been fully vaccinated against COVID-19, and over 201 million people have received at least one dose of vaccine.
Children between the ages of 12 and 15 do not fall under the new vaccine approval, meaning that they will continue to only qualify for COVID-19 vaccination under the emergency use authorization program.
“The FDA’s prescribing information for the vaccine includes its associated risk of myocarditis and pericarditis, two types of heart inflammation that have appeared rarely among people who’ve received the mRNA vaccines, mostly within seven days after the second shot, health officials said,” reports Stat News, a medical news website. “Men under 40 appear to be at higher risk than women and older men, with the highest observed risk in boys age 12 to 17.”
Most of the cases have since been resolved and were only minor, according to the FDA. Federal health officials will require “postmarketing studies” to investigate cases of myocarditis and pericarditis after vaccination. “These studies will include an evaluation of long-term outcomes among individuals who develop myocarditis following vaccination with Comirnaty.”
This is a breaking news story, refresh the page for updates.
.png)
.png)

